11+ Tarveda Therapeutics
In phase Ia dose escalation patients had relapsedrefractory solid tumors. ADCs are complex molecules composed of an antibody linked to.
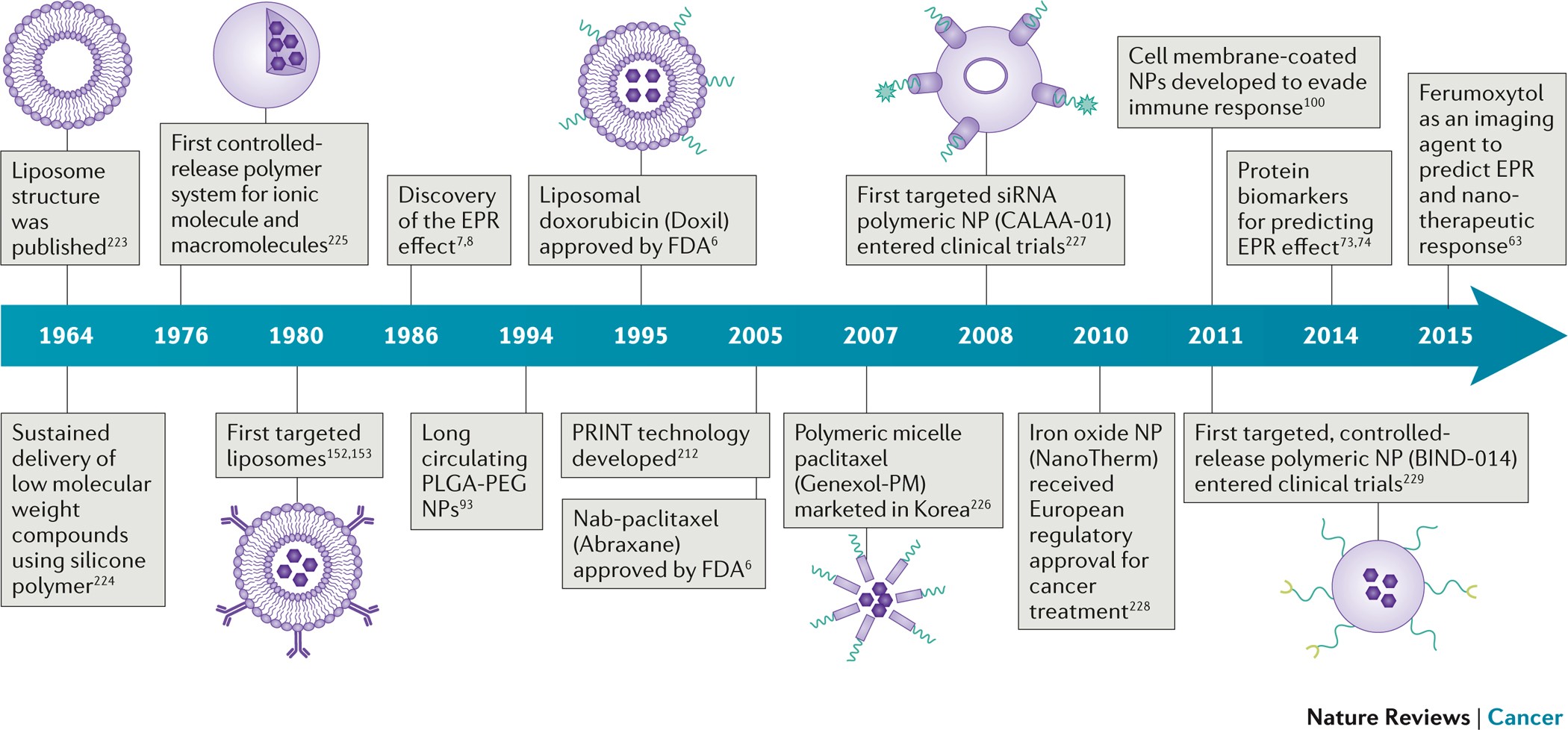
Cancer Nanomedicine Progress Challenges And Opportunities Nature Reviews Cancer
25 subjects received escalating doses of CTL.

. CtDNA refers to the portion of. This was the first-in-human phase 1 dose escalation clinical trial of CTL-002 given IV as monotherapy and in combination with nivolumab in subjects with advanced-stage solid tumors relapsedrefractory to at least one prior anti-PD-1PD-L1 therapy and having exhausted all available therapeutic options. Web Langer has been involved in the founding of numerous biotech and med-tech companies including Semprus Biosciences Frequency Therapeutics Tarveda Therapeutics Microchips Biotech and many more.
Farokhzad previously founded BIND Therapeutics NASDAQ. 2015 Feb 11 Epub ahead of print. Tiragolumab plus atezolizumab was well tolerated with a safety profile generally.
Web He has authored over 180 papers and is an inventor of over 200 issued and pending patents. In phase Ib dose expansion patients had checkpoint. Web Synthetic mRNA represents an exciting cancer vaccine technology for the implementation of effective cancer immunotherapy.
Endovascular therapy for ischemic stroke with perfusion-imaging selection. Web In blood plasma cfDNA typically consists of double-stranded DNA fragments of around 140170 base pairs bp in length that mostly originate from leukocytes 1112. Web Tiragolumab plus atezolizumab showed a clinically meaningful improvement in objective response rate and progression-free survival compared with placebo plus atezolizumab in patients with chemotherapy-naive PD-L1-positive recurrent or metastatic NSCLC.
2022 leading ischemic stroke companies such as athersys inc tarveda therapeutics lumosa therapeutics nc medial research inc acticor biotech meridigen biotech co. Web Epub 2016 Nov 11. Web Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer.
Web 在其公开的管线中肿瘤临床早期的在研产品共计11款不含上市药物的新适应症研究RDC药物便占据了4席足见拜耳对RDC药物的重视. Authors Jinjun Shi 1 Philip W Kantoff 2 Richard Wooster 3 Omid C. Web N Engl J Med.
Medical writing assistance was provided by Liz Leight PhD of Amgen. He is a 2018 Fellow of the National Academy of Inventors. 3 Tarveda Therapeutics Watertown Massachusetts 02472 USA.
This site tracks which universities and pharmaceutical companies are doing this and which arent. In this first randomized phase 3 trial for a KRAS G12C inhibitor oral sotorasib demonstrated superior PFS and ORR compared to intravenous docetaxel and had a more favorable safety profile. He holds over 1300 patents for biotechnologies.
Web Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in the United States with less than half of patients living 5 years following diagnosis. However inefficient in vivo mRNA delivery along with a requirement for immune co-stimulation present major hurdles to achieving anti-tumor therapeutic efficacy. Unlike chemotherapy ADCs are intended to target and kill tumor cells while sparing healthy cellsAs of 2019 some 56 pharmaceutical companies were developing ADCs.
The NCCN Guidelines for Ovarian Cancer provide recommendations for the diagnosis evaluation treatment and follow-up for patients with ovarian fallopian tube. Web The median duration of treatment exposure was 111 months range 00 to 306 in the pembrolizumab group and 57 months range 01 to 396 in the chemotherapy group. BIND acquired by Pfizer Selecta Biosciences NASDAQ.
SELB and Tarveda Therapeutics. Interim overall survival OS analysis from the Phase 3 EMPOWER-Cervical 1 study showed that cemiplimab monotherapy significantly improved OS vs investigators choice IC single-agent chemotherapy chemo in patients pts with RM cervical cancer after progression on first-line 1L platinum-based chemo median follow-up. To evaluate AZD4635 an adenosine A2A receptor antagonist as monotherapy or in combination with durvalumab in patients with advanced solid tumorsPatients and Methods.
Web The median OS was 140 months 95 CI 117 to 161 with T D CT versus 117 months 95 CI 105 to 131 with CT. The PFS and OS benefit with T D CT versus CT was generally consistent with the ITT population across patient subgroups including all those defined by PD-L1. 24-month OS rates were 329 versus 221.
Web By law all clinical trials on the European Union Clinical Trials Register EUCTR must report their results in the registry within a year of completion. Web This Review highlights recent progress in precision therapeutics and drug delivery and identifies opportunities for strategies to improve the therapeutic index of cancer drugs and consequently. Campbell BC et al.
Here we demonstrate a proof-of-concept. 4 King Abdulaziz University Jeddah 21589 Saudi Arabia.

Efficacy And Safety Of Umbralisib Ublituximab U2 And U2 Plus Bendamustine In Patients With Relapsed Or Refractory Diffuse Large B Cell Lymphoma Dlbcl Sciencedirect

Tm2119232d5 Ex99 1img081 Jpg

Pancreatic Ductal Adenocarcinoma Pipeline Analysis 80 Companies Are Working To Improve The Treatment Space Delveinsight Digital Journal
Tarveda Therapeutics Company Information Funding Investors Dealroom Co

Tarveda Therapeutics To Merge With Organovo 3d Printing Media Network The Pulse Of The Am Industry

Cellink Brands Organovo S 3d Bioprinting Patent Lawsuit Invalid 3d Printing Industry

Tarveda Therapeutics Crunchbase Company Profile Funding
Heterogeneity Of Neuroendocrine Transcriptional States In Metastatic Small Cell Lung Cancers And Patient Derived Models Nature Communications

Efficient Blockade Of Locally Reciprocated Tumor Macrophage Signaling Using A Tam Avid Nanotherapy Science Advances

Xencor Culls 2 Bispecifics After Early Phase Data Underwhelm

Overcoming Differential Tumor Penetration Of Braf Inhibitors Using Computationally Guided Combination Therapy Science Advances

Radiation Therapy Primes Tumors For Nanotherapeutic Delivery Via Macrophage Mediated Vascular Bursts Science Translational Medicine

Sudha Kadiyala Phd Newton Centre Massachusetts United States Professional Profile Linkedin

Tarveda Therapeutics Crunchbase Company Profile Funding
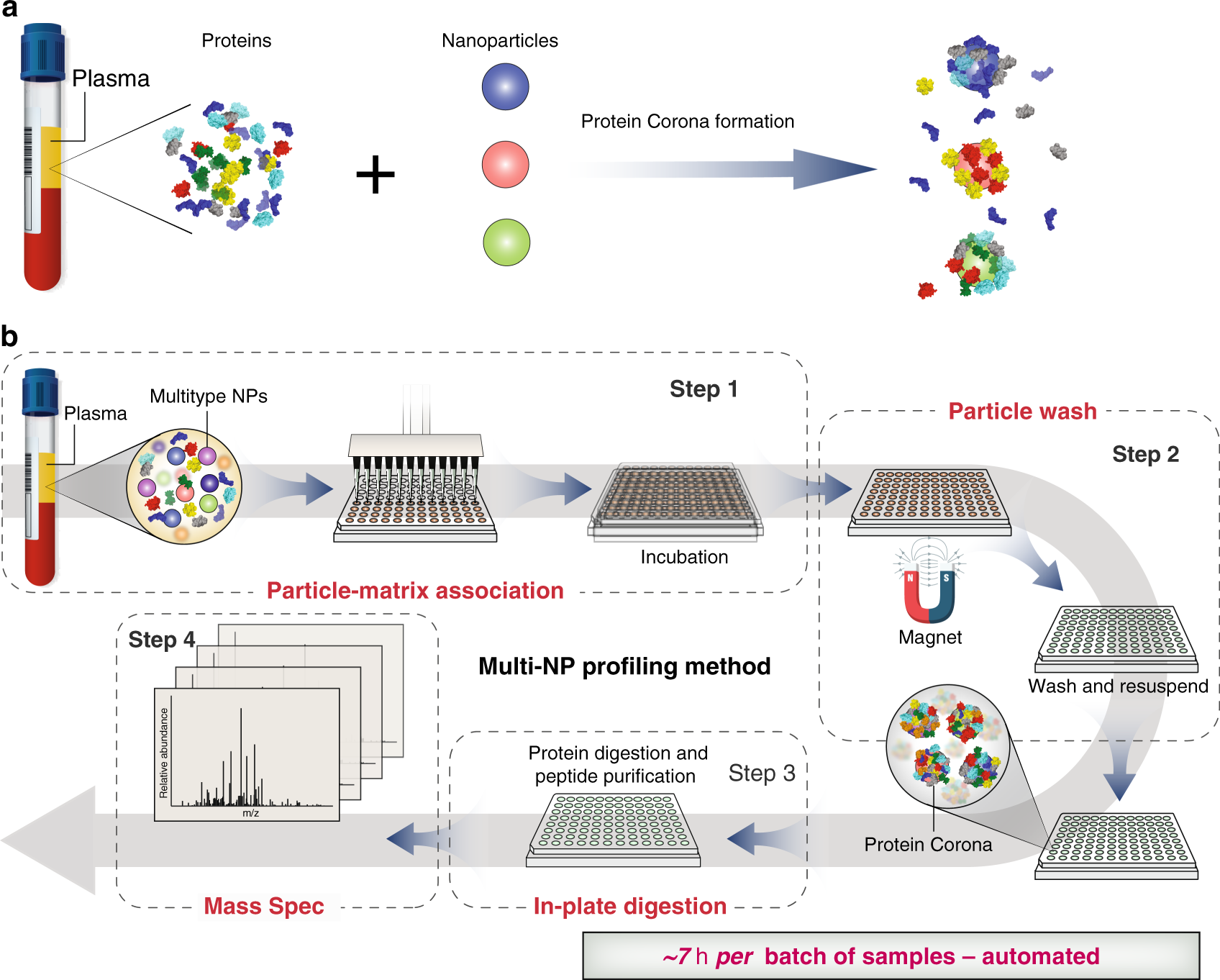
Rapid Deep And Precise Profiling Of The Plasma Proteome With Multi Nanoparticle Protein Corona Nature Communications
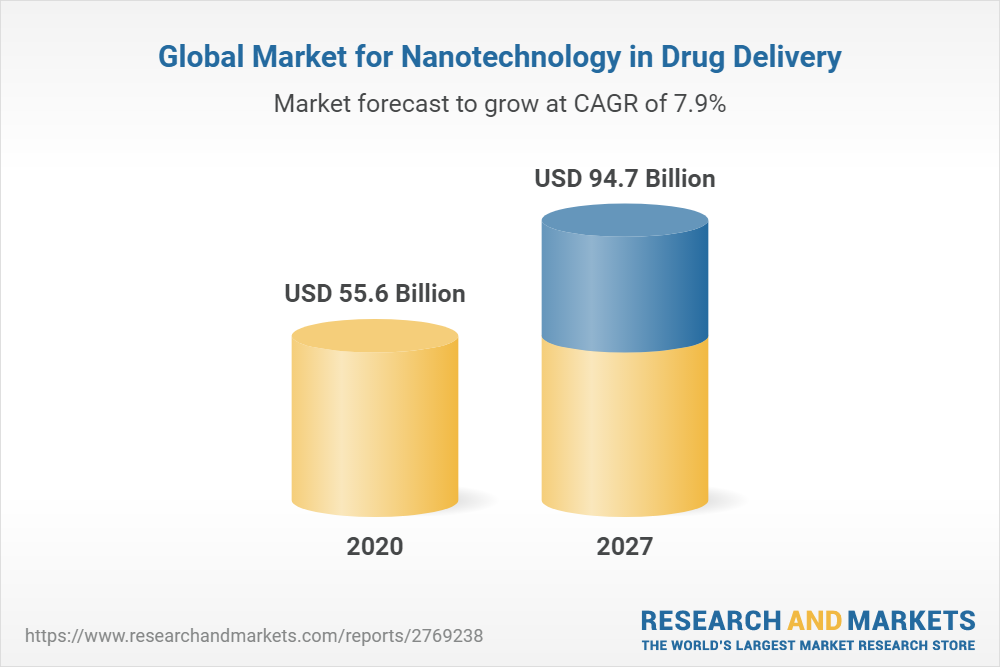
Nanotechnology In Drug Delivery Global Strategic Business Report

Organovo Holdings Inc Merger Prospectus Communication 425